Do you keep up with Joint Commission credentialing standards? If so, you’ve probably noticed they can be about as clear as mud. Fear not, in this article we will take you through everything you need to know about staying compliant with these crucial standards, from the basics to the most granular details that often trip-up people.
Joint Commission Basics
The Foundation of Joint Commission Standards
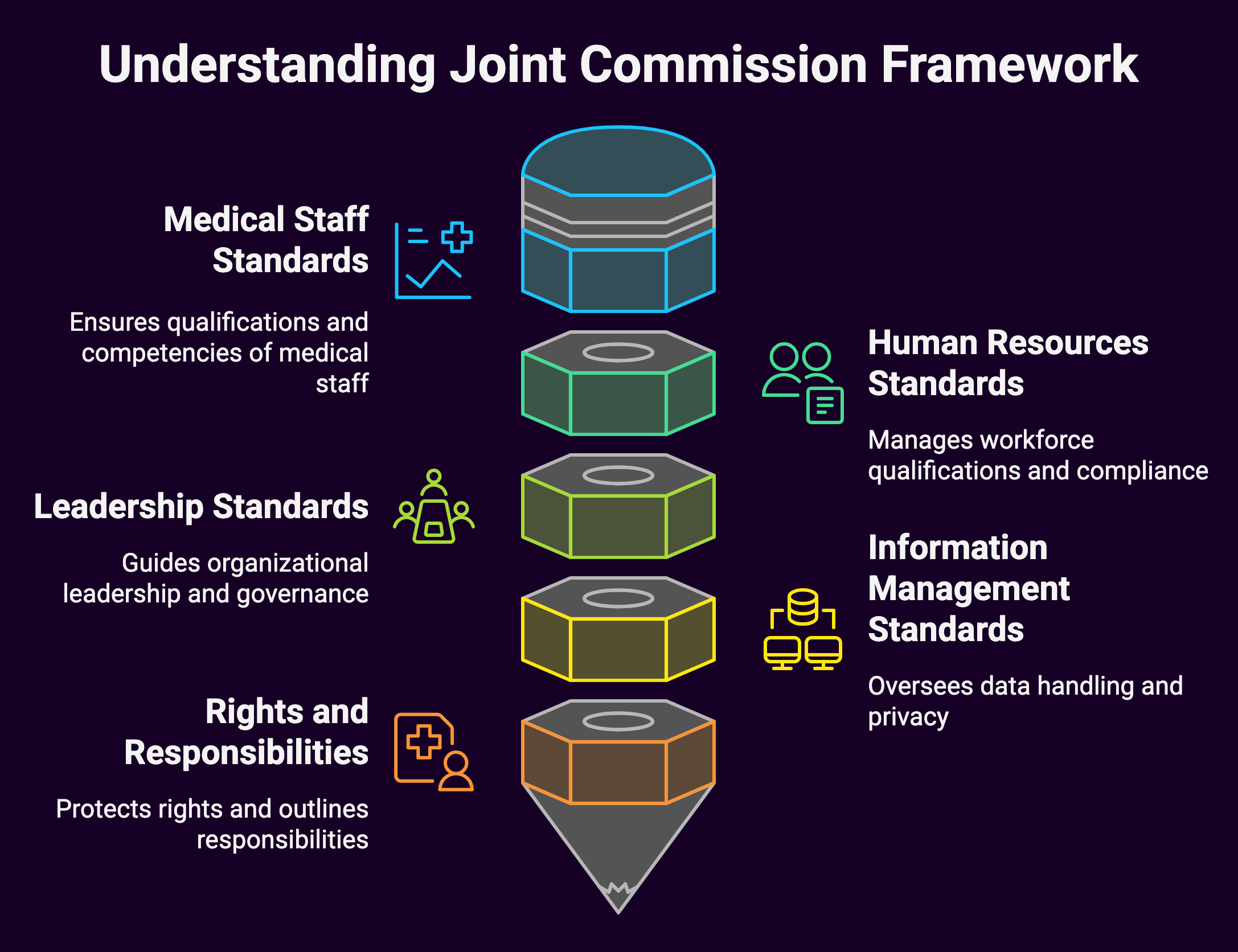
Let’s start with the basics:
Key Components
- Medical Staff (MS) standards
- Human Resources (HR) standards
- Leadership (LD) standards
- Information Management (IM) standards
- Rights and Responsibilities (RI) standards
Regulatory Framework
- Federal requirements alignment
- State law integration
- CMS Conditions of Participation
- Industry best practices
- Evidence-based standards
The Three-Year Survey Cycle
Understanding the survey cycle is crucial:
Pre-Survey Phase
- Self-assessment
- Documentation review
- Policy updates
- Staff education
- Mock surveys
Survey Process
- Document review
- Staff interviews
- Process observation
- Facility tours
- Exit conference
Post-Survey Activities
- Finding resolution
- Action plan development
- Implementation monitoring
- Progress reporting
- Sustained compliance
Core Credentialing Standards
Primary Source Verification
Let’s dive into what needs primary source verification:
Required Elements
- Education and training
- Licensure
- Board certification
- Work history
- Malpractice history
- Criminal background
Verification Timeline
- Initial appointment
- Reappointment cycle
- Ongoing monitoring
- Expiration tracking
- Update requirements
Focused Professional Practice Evaluation (FPPE)
Understanding FPPE requirements:
Implementation Requirements
- New privilege monitoring
- Performance concerns
- Trigger events
- Documentation standards
- Timeline requirements
Documentation Needs
- Criteria development
- Monitoring methods
- Feedback processes
- Resolution documentation
- Follow-up plans
Medical Staff Standards (MS)
MS.06.01.03 – Ongoing Professional Practice Evaluation
Breaking down OPPE requirements:
Essential Elements
- Performance monitoring
- Data collection
- Analysis methods
- Feedback processes
- Action planning
Implementation Strategies
- Metric selection
- Data gathering
- Review processes
- Documentation systems
- Follow-up procedures
MS.06.01.05 – Privileging
Understanding privileging requirements:
Core Requirements
- Criteria development
- Evidence review
- Current competency
- Volume requirements
- Outcome analysis
Documentation Needs
- Application forms
- Supporting evidence
- Committee reviews
- Decision documentation
- Appeals process
Human Resources Standards (HR)
HR.01.02.05 – Primary Source Verification
Getting HR verification right:
Required Elements
- License verification
- Certification checks
- Education confirmation
- Background screening
- Reference checks
Timeline Requirements
- Initial verification
- Renewal timing
- Ongoing monitoring
- Update frequency
- Documentation retention
HR.01.02.07 – Competency Assessment
Understanding competency requirements:
Assessment Components
- Initial evaluation
- Ongoing monitoring
- Skills validation
- Knowledge testing
- Performance review
Documentation Requirements
- Assessment tools
- Result recording
- Action planning
- Follow-up documentation
- Record retention
Documentation Requirements
Essential Documentation
Keep these records pristine:
Provider Files
- Application materials
- Verification results
- Committee minutes
- Privilege forms
- Performance data
Process Documentation
- Policies and procedures
- Assessment criteria
- Review schedules
- Action plans
- Follow-up records
Electronic Systems
Leveraging technology effectively:
System Requirements
- Security features
- Access controls
- Audit trails
- Backup systems
- Integration capabilities
Implementation Considerations
- User training
- Data migration
- Process mapping
- Quality controls
- Maintenance plans
Ongoing Monitoring Requirements
Continuous Compliance
Staying on track:
Monitoring Elements
- License expiration
- Certification status
- Sanction checks
- Performance metrics
- Incident reports
Documentation Needs
- Tracking systems
- Review documentation
- Action records
- Follow-up notes
- Outcome documentation
Performance Monitoring
Keeping tabs on quality:
Data Collection
- Quality metrics
- Patient outcomes
- Peer review
- Patient feedback
- Incident reports
Analysis Requirements
- Trend identification
- Benchmark comparison
- Action planning
- Progress monitoring
- Outcome evaluation
Common Citations and Solutions
Citation 1: Incomplete Files
Solution:
- File audits
- Checklist implementation
- Regular reviews
- Documentation system
- Quality controls
Citation 2: Missing OPPE Data
Solution:
- Data collection systems
- Regular reviews
- Documentation protocols
- Follow-up procedures
- Quality monitoring
Citation 3: FPPE Issues
Solution:
- Clear criteria
- Monitoring systems
- Documentation protocols
- Timeline tracking
- Follow-up procedures
Best Practices for Compliance
Organization and Planning
Stay ahead of the game:
File Management
- Digital systems
- Regular audits
- Update schedules
- Quality checks
- Backup procedures
Process Management
- Clear workflows
- Assignment tracking
- Timeline monitoring
- Quality controls
- Regular reviews
Communication Strategies
Keep everyone in the loop:
Internal Communication
- Regular updates
- Clear protocols
- Documentation systems
- Team meetings
- Progress reports
External Communication
- Provider updates
- Survey readiness
- Compliance reports
- Stakeholder information
- Progress updates
Future Trends and Changes
Technology Integration
Watch for these developments:
Digital Transformation
- Electronic credentialing
- Automated verification
- Integration systems
- Mobile access
- Cloud solutions
AI and Automation
- Data analysis
- Predictive monitoring
- Risk assessment
- Compliance tracking
- Performance evaluation
Regulatory Evolution
Stay ahead of changes:
Standard Updates
- New requirements
- Process changes
- Documentation needs
- Technology standards
- Best practices
Industry Trends
- Telehealth integration
- Remote credentialing
- Virtual surveys
- Digital documentation
- Quality metrics
Practical Implementation Steps
Getting Started
Begin with these steps:
1. Assessment
- Current state review
- Gap analysis
- Resource evaluation
- Timeline development
- Team assembly
2. Planning
- Process development
- System selection
- Training programs
- Implementation schedule
- Quality metrics
3. Implementation
- System setup
- Staff training
- Process rollout
- Monitoring implementation
- Performance tracking
4. Maintenance
- Regular updates
- Process refinement
- Compliance monitoring
- Performance review
- Continuous improvement
Summary: Joint Commission Credentialing Standards
Staying compliant with Joint Commission credentialing standards requires attention to detail, consistent monitoring, and regular updates to processes and procedures. Credentialing standards are dynamic and do morph, particularly as healthcare delivery changes and technology advances. The key to success lies in creating systematic approaches while maintaining flexibility to adapt to changing requirements.

